So here is the summary that you have probably have already heard, Tim Hunt, a Nobel laureate scientist made some very sexist remarks to of all people, a group of female scientists and engineers. He stated men and women shouldn’t work together in the same lab because when they do, you fall in love with them, they fall in love with you, and they cry when you criticize them. I think the man thinks a bit too highly of himself that any women he works with would fall in love with him.
The reaction mocking him, especially on Twitter, has kept my faith in humanity. Women have been tweeting photos of themselves working in the field and lab. Showing how distractingly sexy they are. I tweeted two photos of myself from HAZWOPER training, once in Level A PPE and one in Level B PPE.
Yes I know I am #distractinglysexy in my Level A PPE. The suit totally flatters my curves. pic.twitter.com/LUb5nXx9yO
— Geeky Girl Engineer (@gkygirlengineer) June 11, 2015
Here I am again being #distractinglysexy in Level B PPE. Because who can’t resist a girl with her own supplied air? pic.twitter.com/uCAwdtOm6R
— Geeky Girl Engineer (@gkygirlengineer) June 11, 2015
Those tweets have proved quite popular with the Level A photo thus far getting over 1100 retweets, and the Level B getting over 360 retweets. The tweets have been featured in articles in Buzzfeed, Washington Post, Salon, and Huffington Post UK. The whole thing has been rather surreal honestly. I have been contacted my media outlets to comment. I haven’t, partially because of timing and such.
I don’t even have any photos of me really working in the lab or field that would demonstrate how real work is the complete opposite of distractingly sexy. Well, I guess everyone find different things sexy, but get real. In the first part of my career I worked as a consultant. Typical field work included environmental site assessments where I was directing drillers to get soil and groundwater samples. Gloves, steel-toed boots, jeans, and a t-shirt that was likely going to get dirt on it were my “sexy” look. Then there was the time I was helping to sample a malfunctioning aeration chamber at a wastewater treatment plant in 95°F heat. [The aeration chamber is generally the start of secondary treatment, and thus there should be little to no smell. As this was malfunctioning, try to imagine the smell of raw sewage cooking in the heat.] If you find that situation sexy, well, I don’t think I want to meet you. Then there was the time I was checking on a pilot water treatment plant. Mainly it was a whole lot of sitting around, taking notes, checking valves, and taking some samples by myself. Normally field work involves a lot of sweating really. However, there was one time I was working in the field, again getting soil samples, in New Jersey in the dead of winter. There was no sweating or falling in love. There was just me freezing my butt off and making sure the security guards were in sight. That was a fun job; it was the only time I’ve ever been in a location where safety from crime was an actual issue. Normally the safety issues are the more mundane moving parts, heat, sun, fire ants, and then the one rattlesnake. God bless Texas.
When I was a Ph.D. student, we did our field work at auto body shops measuring the exposure the painters received to a chemical in the clear coat. Basically the shops were loud and smelly with really fun chemicals, and we sat around all day collecting personal air samples, tape strips from their skin after painting, all the urine we could get, and blood at the end of the day. In the hot months, there was sweating. In the cold months, there was shivering. At what point would we be distracting each other with our sexiness? Would the latex gloves and respirators, be the cause? No doubt the painters were falling in love with me because I kept trying to get them to drink more water and begging them for more urine. After the field work was done, I spent the better part of two years or possibly more in the lab analyzing all the urine samples. I analyzed over 400 urine samples, and the analysis was a three day procedure. The first part of the analysis involved adding concentrated sulfuric acid to the urine and then heating it for four hours to 100°C. Yes, nothing says distractingly sexy like urine cooked with acid. Luckily, the lab has hoods and other ventilation methods. Oh, and I shouldn’t leave out the part of asking my lab mates for their urine at times because I used that as unexposed urine from which to make my standards. How I did not fall in love with them while they handed me cups of their own urine, is anyone’s guess.
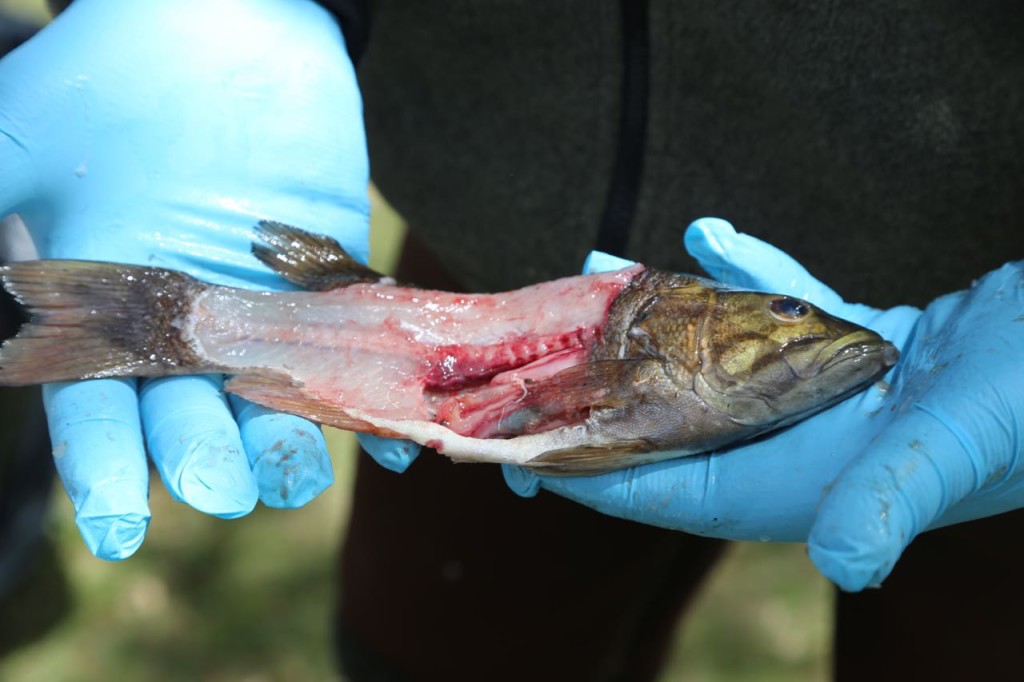
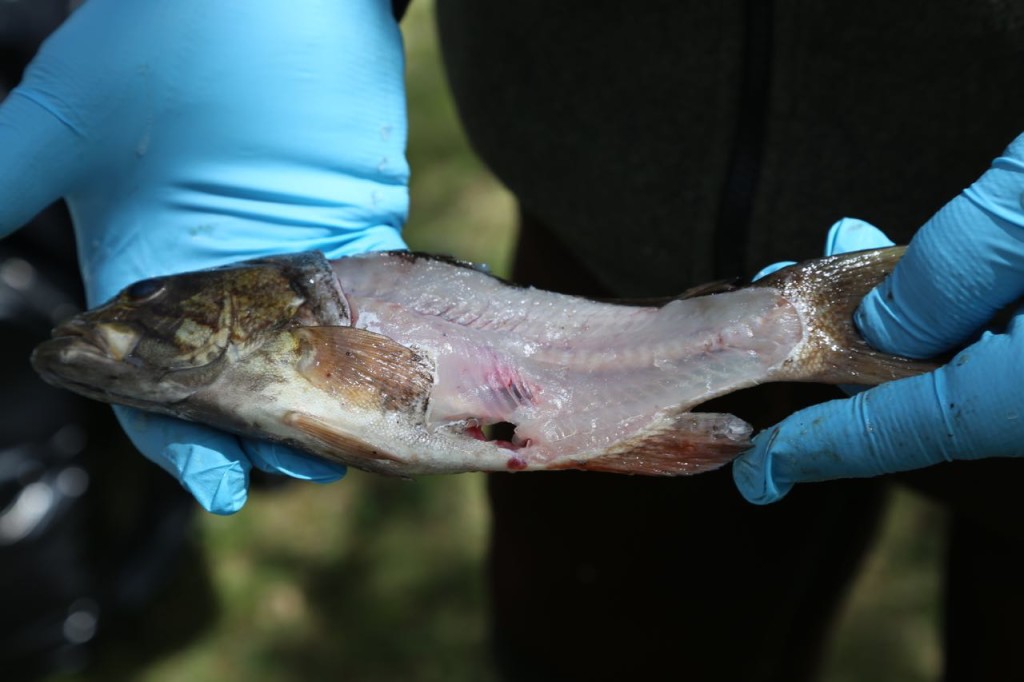
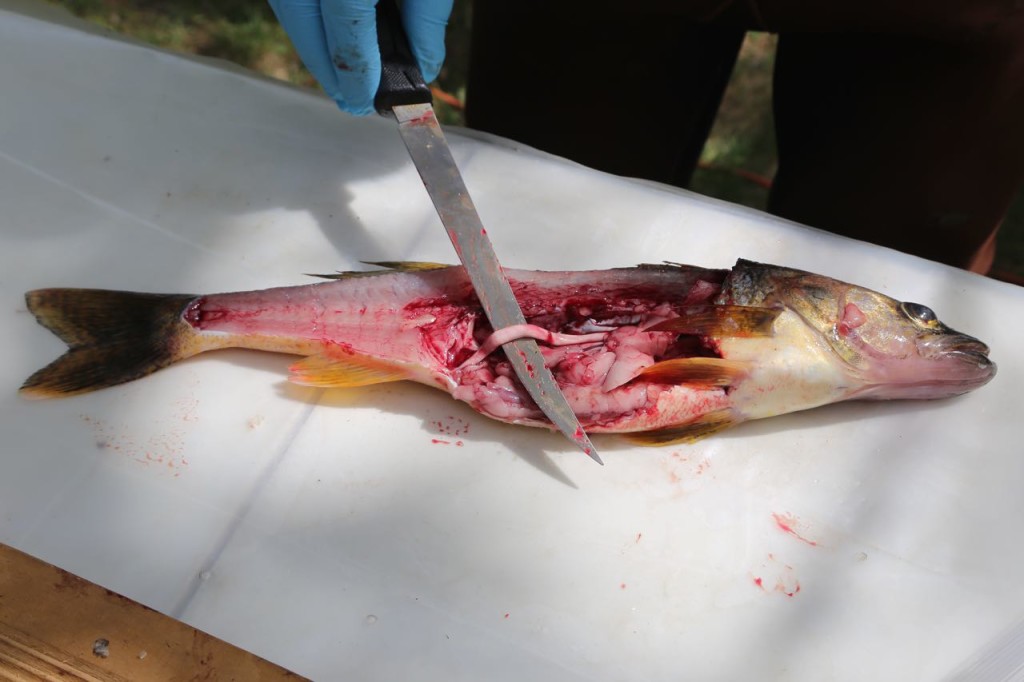
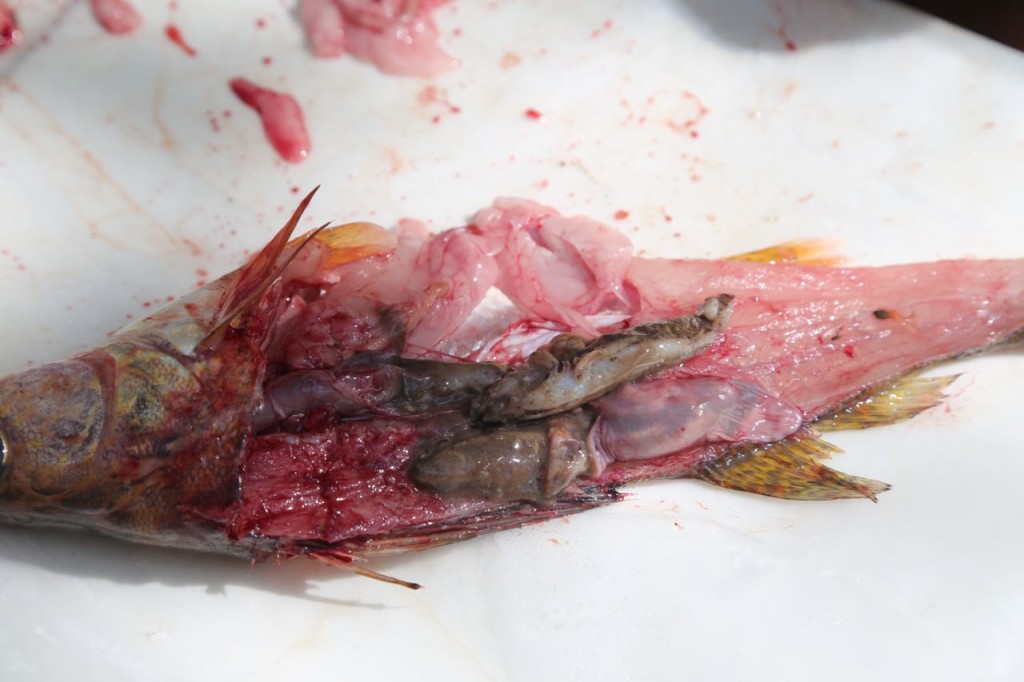
Now, I mainly work in an office. I get into the field every once in a great while. The photos I tweeted are from training, and I have never actually worn that level of PPE for real work. However a couple of weeks ago, I got into the field, and got to help sample fish, then watch a biologist sample them. I did not in fact fall in love with the biologist when he was filleting the fish.